Answer: Option (B) is the correct answer.
Step-by-step explanation:
The given data is as follows.
pH for blood = 7.35,
For carbonic acid,
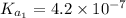
Therefore, calculate the pH of buffer solution as follows.
pH =
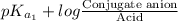
=
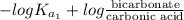
Now, putting the given values into the above formula as follows.
pH =
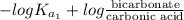
7.35 =
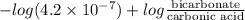
7.35 =
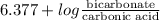
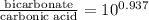
= 9.4
Therefore, we can conclude that the ratio of [bicarbonate]/[carbonic acid] at this pH is 9.4.