Answer:
D - Silver has lower reactivity than hydrogen and cannot replace it
Step-by-step explanation:
If we assume , Ag also reacts just like other metals as
Metal + H₂O ---> Metal Hydroxide + H₂
then Ag forms as
Ag + H2O ----->AgOH +
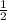 H2
H2
here Ag is converting into Ag⁺ and 2H⁺ are converting into H₂
for reaction Ag --->Ag⁺ + e⁻ E° = -0.799 V
for reaction H⁺ + e⁻ ---->
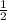 H₂ E° = 0 V
H₂ E° = 0 V
by Adding those 2 above equations net E° will be -0.799 which is negative.As per Faradays Law
ΔG° = -nFE°
here we got E° as negative then ΔG° will be positive
As per thermodynamics if ΔG° is +ve then reaction is not feasible
so, then given reaction does not occur.....