Answer:
The molality of isoborneol in camphor is 0.53 mol/kg.
Step-by-step explanation:
Melting point of pure camphor= T =179°C
Melting point of sample =
 = 165°C
= 165°C
Depression in freezing point =
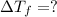
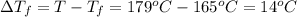
Depression in freezing point is also given by formula:
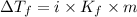
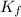 = The freezing point depression constant
= The freezing point depression constant
m = molality of the sample
i = van't Hoff factor
We have:
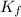 = 40°C kg/mol
= 40°C kg/mol
i = 1 ( organic compounds)
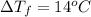
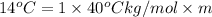
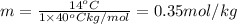
The molality of isoborneol in camphor is 0.53 mol/kg.