Answer:
Because these three have different arrangements of
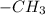
Step-by-step explanation:
All of these alkanes:
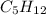 Pentane, Isopentane, and Neopentane despite being homologous have the same chemical formula and their bonding rearranged, with branches of
Pentane, Isopentane, and Neopentane despite being homologous have the same chemical formula and their bonding rearranged, with branches of
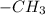 differently placed what gives them different physical and chemical properties such as melting and boiling points different.
differently placed what gives them different physical and chemical properties such as melting and boiling points different.