Answer:
isentropic efficiency = 0.818
Step-by-step explanation:
given data
pressure P1 = 95 kPa
temperature = 27°C
pressure P2 = 600 kPa
temperature = 277°C
to find out
isentropic efficiency of the compressor and exit temperature of the air
solution
we know from ideal gas of properties of air is
Pr1 at 27°C = 1.3860
and h1 at 300 K = 300.19 kJ/kg
and h2 at 550 K = 555.74 kJ/kg
and
we know equation for isentropic process that is
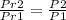 .........................1
.........................1
put here value we get
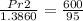
solve we get Pr2
Pr2 = 8.75
by ideal gas of properties of air will be at Pr2
h2s = 508.66
T2s = 505.5 K
'so
isentropic efficiency will be here as
isentropic efficiency =
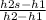
isentropic efficiency =
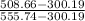
isentropic efficiency = 0.818