Answer:
4P + 5
 → 2
→ 2
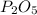
Step-by-step explanation:
There are 4 molecules of phosphorus and 10 molecules of oxygen in the chemical reaction.
The resultant compound is 2
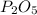 , which is,
, which is,
multiplying 2 with the subscript on P and O gives;
2 × 2 = 4 molecules of P and
2 × 5 = 10 molecules of O
The chemical equation is balanced.