Answer : The heat of the reaction is -221.6 kJ
Explanation :
Heat released by the reaction = Heat absorbed by the calorimeter
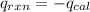
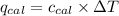
where,
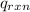 = heat released by the reaction = ?
= heat released by the reaction = ?
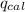 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter
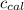 = specific heat of calorimeter =
= specific heat of calorimeter =
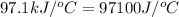
 = change in temperature =
= change in temperature =
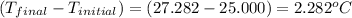
Now put all the given values in the above formula, we get:
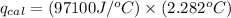
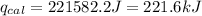
As,
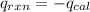
So,
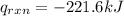
Thus, the heat of the reaction is -221.6 kJ