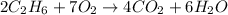Answer :
(1) The balanced chemical reaction will be,
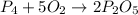
(2) The balanced chemical reaction will be,
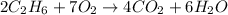
Step-by-step explanation:
Balanced chemical reaction : It is defined as the reaction in which the number of atoms of individual elements present on reactant side must be equal to the product side.
Part 1 :
The given unbalanced chemical reaction is,
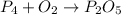
This reaction is an unbalanced chemical reaction because in this reaction number of phosphorous and oxygen atoms are not balanced.
In order to balance the chemical equation, the coefficient '2' put before the
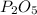 and the coefficient '5' put before the
and the coefficient '5' put before the
 then we get the balanced chemical equation.
then we get the balanced chemical equation.
The balanced chemical reaction will be,
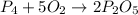
Part 2 :
The given unbalanced chemical reaction is,
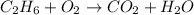
This reaction is an unbalanced chemical reaction because in this reaction number of carbon , hydrogen and oxygen atoms are not balanced.
In order to balance the chemical equation, the coefficient '2' put before the
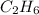 , the coefficient '7' put before the
, the coefficient '7' put before the
 , the coefficient '4' put before the
, the coefficient '4' put before the
 and the coefficient '6' put before the
and the coefficient '6' put before the
 then we get the balanced chemical equation.
then we get the balanced chemical equation.
The balanced chemical reaction will be,