Answer:
1.5 × 10²³ atoms Mn
General Formulas and Concepts:
Math
Pre-Algebra
Order of Operations: BPEMDAS
- Brackets
- Parenthesis
- Exponents
- Multiplication
- Division
- Addition
- Subtraction
Chemistry
Atomic Structure
- Using Dimensional Analysis
- Avogadro's Number - 6.022 × 10²³ atoms, molecules, formula units, etc.
Step-by-step explanation:
Step 1: Define
0.25 mol Mn
Step 2: Identify Conversions
Avogadro's Number
Step 3: Convert
- Set up:
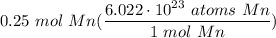
- Multiply:
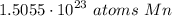
Step 4: Check
Follow sig fig rules and round. We are given 2 sig figs.
1.5055 × 10²³ atoms Mn ≈ 1.5 × 10²³ atoms Mn