Answer:
B) 6.9 × 10⁻⁹
Step-by-step explanation:
HZ is a weak acid that ionizes according to the following generic equation.
HZ(aq) ⇄ H⁺(aq) + Z⁻(aq)
First, we have to calculate the concentration of H⁺ using the pH.
pH = -log [H⁺]
[H⁺] = antilog -pH = antilog -4.93 = 1.17 × 10⁻⁵
Then, we have to calculate the initial concentration of the acid (Ca).
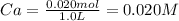
Finally, we can calculate the acid dissociation constant (Ka) using the following expression.
![Ka=([H^(+)]^(2) )/(Ca) =((1.17 * 10^(-5))^(2) )/(0.020) =6.9 * 10^(-9)](https://img.qammunity.org/2020/formulas/chemistry/college/m52zpzks1rh29ohsfhb6k6qvhklnob50f3.png)