Answer:
Step-by-step explanation:
Ca(In)²⁺ + EDTA → Ca(EDTA)²⁺ + In
We use the volume of EDTA consumed in the titration to calculate the moles of Ca⁺² ions:
- 0.012 L * 0.0600 M *
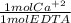 = 7.20x10⁻⁴ mol Ca⁺²
= 7.20x10⁻⁴ mol Ca⁺²
Now we calculate the molarity:
- 7.20x10⁻⁴ mol Ca⁺² / 0.050 L = 0.0144 M
To calculate in ppm, we use the moles of Ca⁺² and convert to mg of CaCO₃:
- 7.20x10⁻⁴ mol Ca⁺² = 7.20x10⁻⁴ mol CaCO₃
- 7.20x10⁻⁴ mol CaCO₃ * 100g/mol *
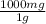 = 72 mg CaCO₃
= 72 mg CaCO₃
Finally, the concentration in ppm is:
- 72 mg CaCO₃ / 0.050L = 1440 ppm