Answer: The pH of the solution when no base is added is 0.311
Step-by-step explanation:
The chemical formula of hydrofluroic acid is HF. It is a monobasic acid.
1 mole of HF produces 1 mole of hydrogen ions and 1 mole of fluoride ions.
To calculate the pH of the solution when no base is added, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/vz65x0ueuj8r8ibqa81zvsbzb2yaetlce4.png)
We are given:
![[H^+]=0.488M](https://img.qammunity.org/2020/formulas/chemistry/college/5tx5vxlyk3ll95vkrhoigt0lpteu1nfk6u.png)
Putting values in above equation, we get:
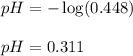
Hence, the pH of the solution when no base is added is 0.311