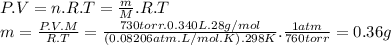Answer:
D. 0.36 g
Step-by-step explanation:
When a gas is collected over water, the total pressure is the sum of the pressure of the gas and the pressure of the water vapor.
Ptotal = Pwater + PN₂
PN₂ = Ptotal - Pwater = 730 torr - 23.76 torr = 706 torr
We can find the mass of N₂ using the ideal gas equation.