Step-by-step explanation:
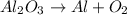
Number of aluminum atoms the reactant side = 2
Number of oxygen atoms the reactant side = 3
Number of aluminum atoms the product side = 1
Number of oxygen atoms the product side = 2
Since, given reaction is not balanced chemical reaction:
So, the balanced chemical reaction is given by:
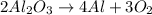
According to reaction,2 moles of aluminum oxide gives 4 moles of aluminum and 3 moles of oxygen gas.