Answer:
The element which is oxidized is = Chlorine
The element which is reduced is = Nitrogen
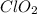 that is chlorine dioxide is acting as a reducing agent.
that is chlorine dioxide is acting as a reducing agent.
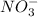 that is nitrate is acting as an oxidizing agent.
that is nitrate is acting as an oxidizing agent.
Step-by-step explanation:
Oxidation reaction is defined as the reaction in which an atom looses its electrons. Here, oxidation state of the atom increases.
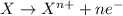
Reduction reaction is defined as the reaction in which an atom gains electrons. Here, the oxidation state of the atom decreases.
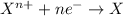
Oxidizing agents are defined as the agents which oxidize other substance and itself gets reduced. These agents undergoes reduction reactions.
Reducing agents are defined as the agents which reduces the other substance and itself gets oxidized. These agents undergoes reduction reactions.
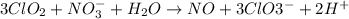
Oxidation state of chlorine in
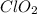 =+4
=+4
Oxidation state of nitrogen in nitrate ion = +5
Oxidation state of nitrogen in NO = +2
Oxidation state of chlorine in in
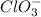 =+7
=+7
The element which is oxidized is = Chlorine
The element which is reduced is = Nitrogen
 that is chlorine dioxide is acting as a reducing agent.
that is chlorine dioxide is acting as a reducing agent.
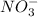 that is nitrate is acting as an oxidizing agent.
that is nitrate is acting as an oxidizing agent.