Answer:
0.3983 mM is the concentration of 2-phosphoglycerate.
Step-by-step explanation:
3-phosphoglycerate ⇄ 2-phosphoglycerate , ΔG° = 4.4 kJ/mol
Concentration of 3-phosphoglycerate at equilibrium = 2.35 mM
Concentration of 2-phosphoglycerate at equilibrium = x
Equilibrium constant of the reaction at 25°C =
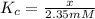
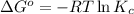
![4400 J/mol=-8.3145 J/mol K* 298.15 K* \ln [(x)/(2.35 mM)]](https://img.qammunity.org/2020/formulas/chemistry/college/6kjnwobqvoik3acmb0vmv2giorgq4oe187.png)
![\ln [(x)/(2.35 mM)]=(4400 J/mol)/(-8.3145 J/mol K* 298.15 K)](https://img.qammunity.org/2020/formulas/chemistry/college/mutszkz4tfkhsj1wcwr17kkwk9nnivzsih.png)
![\ln [(x)/(2.35 mM)]=-1.7750](https://img.qammunity.org/2020/formulas/chemistry/college/ccznnudo9tblahwcpcaeollgawybkan0y4.png)
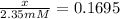
x = 0.3983 mM
0.3983 mM is the concentration of 2-phosphoglycerate.