Step-by-step explanation:
Let us assume that the given data is as follows.
mass of barium acetate = 2.19 g
volume = 150 ml = 0.150 L (as 1 L = 1000 ml)
concentration of the aqueous solution = 0.10 M
Therefore, the reaction equation will be as follows.
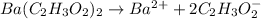
Hence, moles of
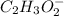 =
=
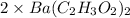 .......... (1)
.......... (1)
As, No. of moles =

Hence, moles of
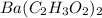 will be calculated as follows.
will be calculated as follows.
No. of moles =

=
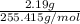 (molar mass of
(molar mass of
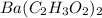 is 255.415 g/mol)
is 255.415 g/mol)
=
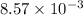
Moles of
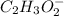 =
=
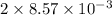
= 0.01715 mol
Hence, final molarity will be as follows.
Molarity =
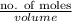
=
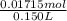
= 0.114 M
Thus, we can conclude that final molarity of barium cation in the solution is 0.114 M.