Answer: Theoretical yield of aluminum sulfate is 3.52 g
Explanation: The balanced reaction of sulfuric acid with aluminum oxide is given by :
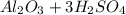 →
→
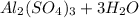
So 1 mole of aluminum oxide reacts with 3 moles of sulfuric acid to form 1 mole of aluminum sulfate and 3 moles of water . Here aluminum oxide is in excess so product will be formed according to sulfuric acid .
Moles of sulfuric acid = mass / molar mass = 3.04 g / 98.07 g/mole
= 0.0309 moles
As we now that 3 moles of sulfuric forms 1 mole of aluminum sulfate .
So 0.0309 moles of sulfuric acid forms = 1 / 3 x 0.0309
= 0.0103 moles .
Now 0.0103 moles of aluminum sulfate = 0.0103 moles x 342.15 g/mole
= 3.52 g
( 342. 15 g /mole = Molar mass of aluminum sulfate )