Answer:
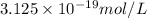 is the concentration of
is the concentration of
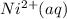 in the solution.
in the solution.
Step-by-step explanation:
![Ni^(2+)(aq) + 3 en\rightleftharpoons [Ni(en)_3]^(2+)(aq)](https://img.qammunity.org/2020/formulas/chemistry/college/qe52fti52kp3tfz220byxaav6dghtnu1dx.png)
Concentration of nickel ion =
![[Ni^(2+)]=x](https://img.qammunity.org/2020/formulas/chemistry/college/8yaxbfi3r2h91zhe3xe4vhluqvlo6i2kyv.png)
Concentration of nickel complex=
![[[Ni(en)_3]^(2+)]=(0.16 mol)/(2 L)=0.08 mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/s6g5ab9vmfieo4j4j8bsy40tje76xzdd8s.png)
Concentration of ethylenediamine =
![[en]=(0.80 mol)/(2 L)=0.40 mol/L](https://img.qammunity.org/2020/formulas/chemistry/college/tq9jilpxmx4dc9rkww4eobx75w9431lzif.png)
The formation constant of the complex =
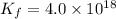
The expression of formation constant is given as:
![K_f=([[Ni(en)_3]^(2+)])/([Ni^(2+)][en]^3)](https://img.qammunity.org/2020/formulas/chemistry/college/qwjeko0c6i245et235n1yjwy0kbqpheu1s.png)
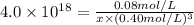
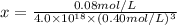
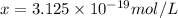
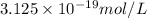 is the concentration of
is the concentration of
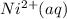 in the solution.
in the solution.