Step-by-step explanation:
Let us assume that the solution contains both
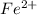 and
and
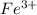 which has a cell voltage of 0.719 V.
which has a cell voltage of 0.719 V.
Therefore, voltage of cell contains both Ag/AgCl reference electrode where
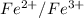 electrode is 0.719 V.
electrode is 0.719 V.
As,
 = 0.719 V
= 0.719 V
It is known that potential of the silver-silver chloride reference electrode is 0.197 V.
Hence,
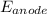 = 0.197 V. Now, calculate
= 0.197 V. Now, calculate
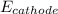 as follows.
as follows.
 = 0.719 V
= 0.719 V
 = 0.719 V
= 0.719 V
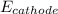 = 0.916 V
= 0.916 V
Now, voltage of the cell that contains both calomel reference electrode and
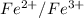 electrode as follows.
electrode as follows.
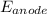 = calomel electrode = 0.241 V
= calomel electrode = 0.241 V
Voltage of cell =

= 0.916 V - 0.241 V
= 0.675 V
Thus, we can conclude that 0.675 V is the new voltage.