Step-by-step explanation:
The given data is as follows.
Mass of ice dropped = 325 g
Initial temperature =
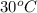 = (30 + 273) K = 303 K
= (30 + 273) K = 303 K
Final temperature =
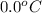 = (0 + 273) K = 273 K
= (0 + 273) K = 273 K
Now, using density of water calculate the mass of ice as follows.
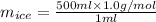
= 500 g
As the relation between heat energy, specific heat and change in temperature is as follows.
Q =
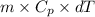
=
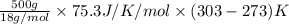
= 62750 J
Also, relation between heat energy and latent heat of fusion is as follows.
Q = m L
=
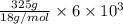
= 108300 J
Therefore, we require
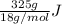 heat but we have 40774.95 J.
heat but we have 40774.95 J.
So,
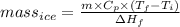
=
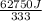
= 188.4 g
Hence, the mass of ice = 325 g - 188.4 g
= 137 g
Therefore, we can conclude that 137 g of ice will still be present when the contents of the pitcher reach a final temperature.