Answer:
∆E = 7.9 × 10⁷ J
Step-by-step explanation:
The air absorbs 1.3 × 10⁸ J of heat (Q), that is, Q = 1.3 × 10⁸ J.
The work (W) done by the expansion of the gas can be calculated using the following expression.
W = -P . ∆V
where,
P is the external pressure
∆V is the change in the volume
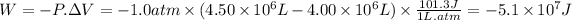
The change in the internal energy (∆E) can be calculated using the following expression.
∆E = Q + W
∆E = 1.3 × 10⁸ J + (-5.1 × 10⁷ J) = 7.9 × 10⁷ J