Answer:
16.7 days
Step-by-step explanation:
We are given;
A radioactive isotope Iodine-131`
The decay rate is 0.138 d⁻¹
The percent decayed is 90%
We are suppose to calculate the number of days for the decay.
![In(([A_(0)])/([A]))=kt](https://img.qammunity.org/2020/formulas/chemistry/college/dnjrd7uwro98tkiq66ianyms1ypeffa3d7.png)
Where,
![[A_(0)]](https://img.qammunity.org/2020/formulas/chemistry/college/b7ietxy3u7wo7f2agj5g2qunz02w0zhvjg.png) is the initial concentration and
is the initial concentration and
![[A]](https://img.qammunity.org/2020/formulas/chemistry/college/ey5pxctwpmy356s81f6qc2pjl6oveugako.png) is the new concentration.
is the new concentration.
![t=In(([A_(0)])/([A]))/k](https://img.qammunity.org/2020/formulas/chemistry/college/fyoj9bf8g8pq3jcxs6wo935k42h3sf5c52.png)
Assuming the initial concentration is x, then the final concentration after 90% decay will be 0.10x
Therefore;
![t=In(([x])/([0.10x]))((1)/(0.138))](https://img.qammunity.org/2020/formulas/chemistry/college/5x9fluwfvunq83kms6kqtjpr4ofr25ak3v.png)
![t=In(([1])/([0.10]))((1)/(0.138))](https://img.qammunity.org/2020/formulas/chemistry/college/dl6p6j7uy8ti3b67425m9n6unafsn02avs.png)
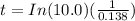
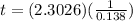
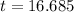
Time = 16.7 days
Therefore, it will take 16.7 days for 90% of I-131 to decay to Xe-131