Answer:
The molarity of a solution prepared by dissolving 10.7 g NaI in 0.250 L is 0.286 mol/L
Step-by-step explanation:
Molarity M is defined as the number of moles of solute that are dissolved in a certain volume, generally expressed in liters:
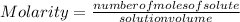
Then you must first know how many moles represent 10.7 g of NaI. For that you must know the mass of the compound, that is, the mass that contains 1 mol of NaI. For that you know that:
- Na: 23 g/mol
- I: 126.9 g/mol
Then:
NaI: 23 g/mol + 126.9 g/mol = 149.9 g/mol
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
a ⇒ b
c ⇒ x
So
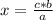
Then you can apply a rule of three to know how many moles represent 10.7 g of NaI as follows: if 149.9 g are contained in one mole of NaI, 10.7 g are contained in how many moles of the compound?
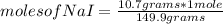
moles of NaI= 0.0714
Then the molarity is
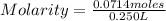
Molarity=0.286
Finally, the molarity of a solution prepared by dissolving 10.7 g NaI in 0.250 L is 0.286 mol/L