Answer : The correct option is, (A) 446 torr
Solution :
According to the Dalton's law, the total pressure of the gas is equal to the sum of the partial pressure of the mixture of gasses.
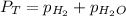
where,
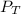 = total partial pressure = 0.610 atm = 463.6 torr
= total partial pressure = 0.610 atm = 463.6 torr
conversion used : (1 atm = 760 torr)
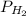 = partial pressure of hydrogen gas = ?
= partial pressure of hydrogen gas = ?
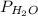 = partial pressure of water vapor = 18 torr
= partial pressure of water vapor = 18 torr
Now put all the given values is expression, we get the partial pressure of the hydrogen gas.
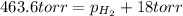
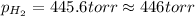
Therefore, the partial pressure of
 gas is, 446 torr
gas is, 446 torr