Answer:
T_f= 77.58° C
Step-by-step explanation:
from simple calorimetry we can write that
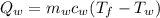
and
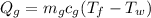
Where
Q_w = heat content of water
Q_g= heat content of glass
m_g= mass of glass
m_w= mass of water
T_f= final temp
T_w= temp of water
T_g= temp of glass
m_w =mass of water
m_g= mass of glass
The specific heat of glass is 0.2 cal/g · ◦ C and of water 1 cal/g · ◦ C.
Now in given case
Q_w+Q_g=0
therefore
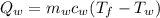 +
+
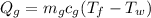 =0
=0
⇒T_f=
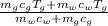
putting values we get
T_f=
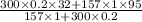
T_f= 77.58° C