Answer:
age of bone is 1756 years
Explanation:
The amount of carbon-14 present in animal bones after t years
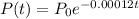
P(t) is the carbon present
19% has lost. so carbon present is 100-19 = 81% present
out of 100 81 is presents
so P0 is 100
P(t) is 81
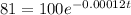
divide by 100 on both sides
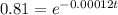
take ln on both sides
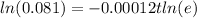
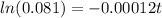
divide both sides by -0.00012
t=1756.0085
age of bone is 1756 years