Answer:
85.46 grams of sodium carbonate would be required for removal of essentially all of the calcium ion from 750 L of solution containing 43 mg calcium ion per liter.
Step-by-step explanation:
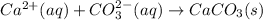
Mass of calcium ions in solution = 43 mg/L = 0.043 g/L (1 mg = 0.001 g)
Volume of solution = 750 L
Mass of calcium ions in 750 L solution = 0.043 g/L × 750 L =32.25 g
Moles of calcium ions =
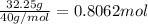
According to reaction, 1 mol of calcium ion reacts with 1 mol of carbonate ions
Then 0.8062 mol of calcium ion will react with:
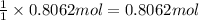 carbonate ions.
carbonate ions.
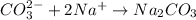
1 mol of carbonation ions combines with 2 moles of sodium ions to form 1 mol sodium carbonate.
Then moles of sodium carbonate formed 0.8062 mol of carbonate ions will:
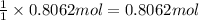
Mass of 0.8062 moles of sodium carbonate :
0.8062 mol × 106 g/mol= 85.46 g
85.46 grams of sodium carbonate would be required for removal of essentially all of the calcium ion from 750 L of solution containing 43 mg calcium ion per liter.