Answer : The volume of gas at STP produced are 45.0 L
Explanation :
First we have to calculate the moles of ammonium perchlorate.
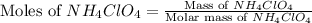
Molar mass of
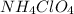 = 117 g/mole
= 117 g/mole
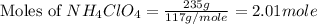
Now we have to calculate the moles of
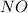 gas produced during the reaction.
gas produced during the reaction.
The balanced chemical reaction is,
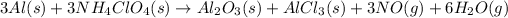
From the reaction we conclude that,
As, 3 moles of
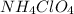 produces 3 moles of NO gas
produces 3 moles of NO gas
So, 2.01 moles of
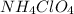 produces 2.01 moles of NO gas
produces 2.01 moles of NO gas
Now we have to calculate the volume of gas at STP.
At STP, 1 mole of substance occupy 22.4 L volume of gas.
As, 1 mole of NO gas occupy 22.4 L volume of gas.
So, 2.01 mole of NO gas occupy 2.01 × 22.4 L = 45.0 L volume of gas.
Therefore, the volume of gas at STP produced are 45.0 L