Answer : The value of
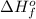 for the reaction is, -640 KJ/mole
for the reaction is, -640 KJ/mole
Explanation :
The steps involved in the formation of
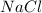 :
:
(1) Conversion of gaseous sodium atoms into gaseous sodium ions.
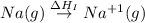
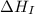 = ionization energy of sodium = 496 kJ/mol
= ionization energy of sodium = 496 kJ/mol
(2) Conversion of gaseous chlorine atoms into gaseous chlorine ions.
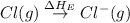
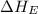 = electron affinity energy of chlorine = -349 kJ/mol
= electron affinity energy of chlorine = -349 kJ/mol
(3) Conversion of gaseous cations and gaseous anion into solid sodium chloride.
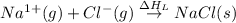
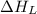 = lattice energy of sodium chloride (always negative) = -787 kJ/mol
= lattice energy of sodium chloride (always negative) = -787 kJ/mol
To calculate the overall energy the equation used will be:
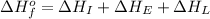
Now put all the given values in this equation, we get:
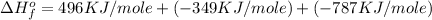
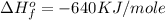
Therefore, the value of
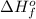 for the reaction is, -640 KJ/mole
for the reaction is, -640 KJ/mole