Answer: The cost is coming out to be $ 1.25
Step-by-step explanation:
To calculate the number of moles for given molarity, we use the equation:
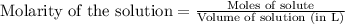
Molarity of HCl solution = 0.4 M
Volume of solution = 0.5 L
Putting values in equation 1, we get:
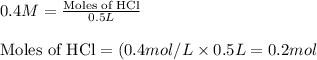
The chemical equation for the reaction of HCl and calcium carbonate follows:
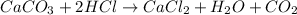
By Stoichiometry of the reaction:
2 moles of HCl reacts with 1 mole of calcium carbonate
So, 0.2 moles of HCl will react with =
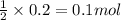 of calcium carbonate
of calcium carbonate
To calculate the mass of calcium carbonate for given moles, we use the equation:

Molar mass of calcium carbonate = 100 g/mol
Moles of calcium carbonate = 0.1 moles
Putting values in equation 1, we get:

- Calculating the mass of calcium carbonate in 1 container:
We are given:
One container contains eighty 1 g of tablets, this means that in total 80 g of tablets are there.
Every container has 40 % calcium carbonate.
Mass of calcium carbonate in 1 container = 40 % of 80 g =

- Calculating the containers for amount of calcium carbonate that neutralized HCl by using unitary method:
32 grams of calcium carbonate is present in 1 container
So, 10 g of calcium carbonate will be present in =
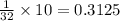 container
container
- Calculating the cost of turns:
1 container of turns costs $4
So, 0.3125 containers of turns will cost =
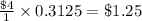
Hence, the cost is coming out to be $ 1.25