Answer : The unknown diatomic gas is chlorine

Explanation :
Density : It is defined as the mass contained per unit volume.
Formula used for density :

Given :
Density of unknown diatomic gas = 3.164 g/L
Volume of gas at STP = 22.4 L
Now put all the given values in the above formula, we get the mass of the unknown diatomic gas.
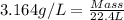
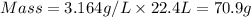
Thus, the mass of unknown diatomic gas is 70.9 grams that means the unknown diatomic gas will be, chlorine

Hence, the unknown diatomic gas is chlorine
