Answer : The final volume of will be 10.5 liters.
Explanation :
Formula used :
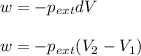
where,
w = work done = -865 J = -8.54 L.atm
Conversion used : (1 L.atm = 101.3 J)
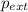 = external pressure = 635 mmHg = 0.836 atm
= external pressure = 635 mmHg = 0.836 atm
Conversion used : (760 mmHg = 1 atm)
 = initial volume of gas = 0.290 L
= initial volume of gas = 0.290 L
 = final volume of gas = ?
= final volume of gas = ?
Now put all the given values in the above formula, we get :
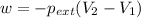
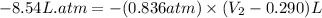
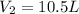
Therefore, the final volume of will be 10.5 liters.