Answer:
1170.43 m³
Step-by-step explanation:
We are given;
- Initial pressure, P1 as 808 kPa
- Initial temperature, T1 as 585 K
- Initial volume, V1 as 295 m³
- New pressure, P2 as 102 kPa
- New temperature, T2 as 293 K
We are required to find the new volume;
- We are going to use the combined gas law
- According to the gas law;
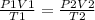
- Thus, rearranging the formula;
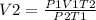
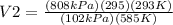
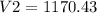
Therefore, the volume is 1170.43 m³