Answer:
The percent yield is 66.7%
Step-by-step explanation:
1. The balanced chemical reaction to obtain carbon dioxide from the methane is:
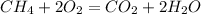
2. Calculathe the theoretical quantity of carbon dioxide.
As the problem says that the limiting reagent is the methane, all the calculations will be made from this quantity:
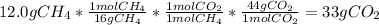
3. Calculate the percent yield:
Percent yield =
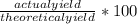
Percent yield =
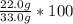
Percent yield = 66.7%