1.74 moles
Step-by-step explanation:
A mole of a substance is defined as:
The mass of substance containing the same number of fundamental units as there are atoms in exactly 12.000 g of
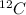 .
.
Any substance has
 atoms in one mole of that substance.
atoms in one mole of that substance.
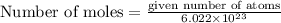
Given number of atoms are

So,
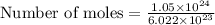 =
=
 moles
moles