Answer:
The wavelength the light emitted by a hydrogen atom during a transition is 1006 nm.
Step-by-step explanation:
By using Rydberg's Equation we cab determine the wavelength of the light:
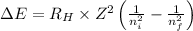
Where,
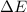 = Energy difference
= Energy difference
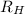 = Rydberg's Constant
= Rydberg's Constant
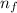 = Final energy level
= Final energy level
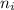 = Initial energy level
= Initial energy level
We have :
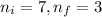 , Z = 1
, Z = 1
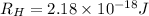
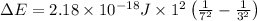
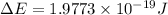
Now by using Plank's equation we can determine the wavelength of the light emitted.
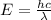
E = Energy of the emitted light
h = Planck's constant =
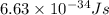
c = speed of light =
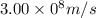
For the given transition the energy of the light = E
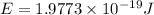
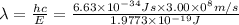
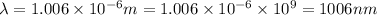
The wavelength the light emitted by a hydrogen atom during a transition is 1006 nm.