Answer:
710.79 Joules
898.56 Joules
264.45 K
172.2 K
Step-by-step explanation:
W = Work done
Q = Heat added = 1248 J
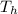 = Temperature of hot reservoir = 615 K
= Temperature of hot reservoir = 615 K
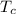 = Temperature of cold reservoir
= Temperature of cold reservoir
Efficiencies of engine A and B
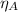 = 0.57
= 0.57
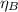 = 0.72
= 0.72
Efficiency is given by
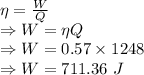
Work done by engine A is 710.79 Joules
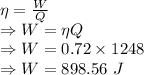
Work done by engine B is 898.56 Joules
Efficiency
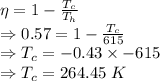
The temperature of the cold reservoir is 264.45 K
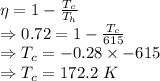
The temperature of the cold reservoir is 172.2 K