Answer:
The rate of appearance of hydrogen gas is 0.095 M/s.
Step-by-step explanation:
Rate of the reaction is the change in concentration of of any one of the reactants or products per unit time.
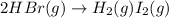
Given:
The rate of disappearance of HBr =
![-(d[HBr])/(dt)=0.190 M/s](https://img.qammunity.org/2020/formulas/chemistry/high-school/r6kdapogbywtr0taw6qnp2sucejfi4ypoc.png)
Rate of the reaction is given by:
![R=-(1)/(2)(d[HBr])/(dt)=(1)/(1)(d[H_2])/(dt)](https://img.qammunity.org/2020/formulas/chemistry/high-school/fruro7rjw0vehyuzq6xzsfgwwvzkyq6msb.png)
![R=-(1)/(2)(d[HBr])/(dt)=(1)/(2)* 0.190 M/s=0.095 M/s](https://img.qammunity.org/2020/formulas/chemistry/high-school/5dayb6yyfx0nboivfshp7o8e3b6j17tti1.png)
Rate of appearance of the hydrogen gas:
![(d[H_2])/(dt)=R =0.095 M/s](https://img.qammunity.org/2020/formulas/chemistry/high-school/5frd8l2vmkri5oyf5um97inoeiv1trtoor.png)
The rate of appearance of hydrogen gas is 0.095 M/s.