Answer:
1,033.56 grams of carbon dioxide was emitted into the atmosphere.
Step-by-step explanation:
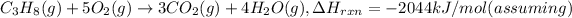
Energy absorbed by pork,E =
 (assuming)
(assuming)
Total energy produced by barbecue = Q
Percentage of energy absorbed by pork = 10%
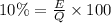
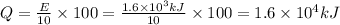
Since, it is a energy produced in order to indicate the direction of heat produced we will use negative sign.
Q =
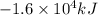
Moles of propane burnt to produce Q energy =n
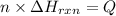
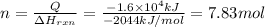
According to reaction , 1 mol of propane gives 3 moles of carbon dioxide. then 7.83 moles of will give:
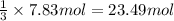 carbon dioxide gas.
carbon dioxide gas.
Mass of 23.49 moles of carbon dioxide gas:
23.49 mol × 44 g/mol =1,033.56 g
1,033.56 grams of carbon dioxide was emitted into the atmosphere.