Answer:
NaCl is more soluble in NH3 and I2 is more soluble in C7H16
Step-by-step explanation:
Polar substances are likely to dissolve in polar solvents. For example, ionic compounds, which are very polar, are often soluble in the polar solvent water. Nonpolar substances are likely to dissolve in non-polar solvents.For example, ionic compounds are insoluble in hexane.
In the above example NaCl is a polar compound made up of ions
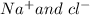 so it is soluble in polar compounds like ammonia (NH3),where as I2 is a non-polar solid so it is soluble in non-polar solvents like n-heptane(C7H16).
so it is soluble in polar compounds like ammonia (NH3),where as I2 is a non-polar solid so it is soluble in non-polar solvents like n-heptane(C7H16).