Answer:
The order of bowls from coolest to warmest based on their final temperatures will be:
Bowl B < Bowl A < Bowl C
Step-by-step explanation:
Same amount of heat is added to each bowl = Q
Bowl -A:
Mass of the water ,m= 20 g
Initial temperature of the water =

Final temperature of the water =

Heat capacity of the water= c = 4.186 J/g°C
Change in temperature of the water =
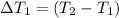
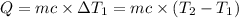
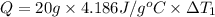
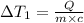
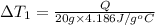 ..[1]
..[1]
Bowl -B:
Mass of the water ,m' = 40 g
Initial temperature of the water =

Final temperature of the water =
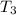
Heat capacity of the water= c = 4.186 J/g°C
Change in temperature of the water =
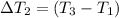
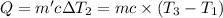
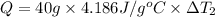
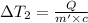
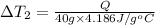 ..[2]
..[2]
Bowl -C:
Mass of the mercury ,m''= 20 g
Initial temperature of the mercury =

Final temperature of the mercury =
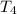
Heat capacity of the mercury= c' = 0.140 J/g°C
Change in temperature of the water =
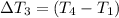
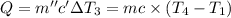
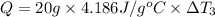
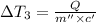
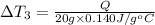 ..[3]
..[3]
Taking ratio of [1] , [2],and [3]
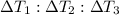
=
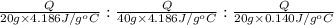
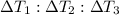 = 1 : 0.5 : 29.9
= 1 : 0.5 : 29.9
As substance with larger value in change in temperature will have higher temperature on addition of energy. So, the order of bowls from coolest to warmest based on their final temperatures will be:
Bowl B < Bowl A < Bowl C