Answer:
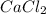
Step-by-step explanation:
Principle Quantum Numbers : It describes the size of the orbital and the energy level. It is represented by n. Where, n = 1,2,3,4....
Halogen with n=3 is chlorine.
Alkaline earth metal with n = 4 is calcium.
An ionic bond is formed when an element completely transfers its valence electron to another element.
Electronic configuration of calcium
![[Ca]=1s^22s^22p^63s^23p^64s^2](https://img.qammunity.org/2020/formulas/chemistry/high-school/lvvm0c58rxn9wtk54m0yj63em436j9pg4q.png)
calcium atom will loose one electron to gain noble gas configuration and form calcium cation with +2 charge.
![[Ca^(2+)]=1s^22s^22p^63s^23p^6](https://img.qammunity.org/2020/formulas/chemistry/high-school/tgh352vz6ni0q9vkz0gegdru37ez3j6ors.png)
Electronic configuration of chlorine:
![[Cl]=1s^22s^22p^63s^23p^5](https://img.qammunity.org/2020/formulas/physics/high-school/htzaac8frg5kul80i9q5iotmnda8y76bas.png)
Chlorine atom will gain one electron to gain noble gas configuration and form chloride ion with -1 charge.
![[Cl^-]=1s^22s^22p^63s^23p^6](https://img.qammunity.org/2020/formulas/physics/high-school/4asj1vyjlhxcwpxow5ijahwuue2vem3hf3.png)
Here
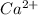 cation and chloride
cation and chloride
 anion combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
anion combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
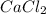 .
.