Answer:
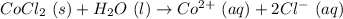
Step-by-step explanation:
Cobalt chloride is a solid and water is a liquid. When we dissolve cobalt chloride in water, the solid dissociates into ions giving positive cobalt ions and negatively charged chlorine ions.
The result of the reaction is thus an aqueous solution containing the ions of the solid and the molecules of water.
The reaction is shown below:
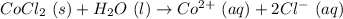
The above reaction must be balanced, so we need to write 2 as coefficient for negatively charged chlorine ions.
The above reaction tells us that when a molecule of cobalt chloride (solid) is reacted with a molecule of water (liquid), we get an aqueous solution of one positively charged Cobalt ion and 2 negatively charged chlorine ions.