Step-by-step explanation:
The given data is as follows.
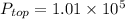
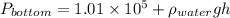
As, density =
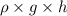
Now, putting the given values into the above formula as follows.
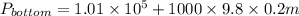
=
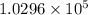
According to ideal gas equation, PV = nRT
And, in the given case PV = nRT = constant
Hence, calculate the volume ratio of top and bottom as follows.
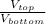 =
=
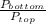
=
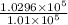
= 1.019
Thus, we can conclude that the ratio of the bubble’s volume at the top to its volume at the bottom is 1.019.