Answer:
The final volume of the gas is 36.1 L.
Step-by-step explanation:
Given:
Initial pressure of the gas is,
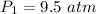
Final pressure of the gas is,
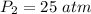
Initial volume of the gas is,
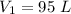
Final volume of the gas is,
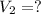
Here, we shall use Boyle's Law which states that for a process under constant temperature, the pressure of the gas changes inversely with the change in volume.
Here, the pressure is increased. So, the volume of the gas is decreased.
Therefore, as per Boyle's Law:
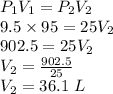
So, the final volume of the gas is 36.1 L.