Answer:
5.63 atm
Step-by-step explanation:
P1 = 5 atm
P2 = ?
T1 = 5 °C = 5 + 273 = 278 K
T2 = 40 °C = 273 + 40 = 313 K
Let the pressure is P2.
Use
Pressure is directly proportional to the absolute temperature as the volume remains constant.
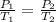
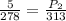
P2 = 5.63 atm
Thus, the pressure is 5.63 atm.