Answer:
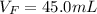
Step-by-step explanation:
Hello,
In this case, we firstly must compute the volume of the piece of metal by using its density as shown below:
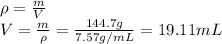
Then, by adding the initial volume of water and the volume of the metal, one obtains the final volume:
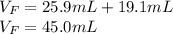
Best regards.