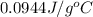Answer :
(a) The heat released by the metal is -312.48 J
(b) The specific heat of the metal is
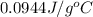
Explanation :
For part A :
Heat released by the metal = Heat absorbed by the calorimeter + Heat absorbed by the water
![q=[q_1+q_2]](https://img.qammunity.org/2020/formulas/chemistry/college/3yqkquw9xt0urtwyu8qfu9kf65yvlx00r8.png)
![q=[c_1* \Delta T+m_2* c_2* \Delta T]](https://img.qammunity.org/2020/formulas/chemistry/college/z4j1tykfisn6ks4ttchepg3letm6np6k8o.png)
where,
q = heat released by the metal
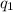 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter
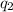 = heat absorbed by the water
= heat absorbed by the water
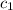 = specific heat of calorimeter =
= specific heat of calorimeter =
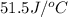
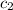 = specific heat of water =
= specific heat of water =
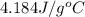
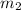 = mass of water = 50.0 g
= mass of water = 50.0 g
 = change in temperature =
= change in temperature =
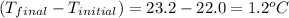
Now put all the given values in the above formula, we get:
![q=[(51.5J/^oC* 1.2^oC)+(50.0g* 4.184J/g^oC* 1.2^oC)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/wad44szjp8bjuvbqtvak5jr651v3bs3a3q.png)
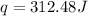
Thus, the heat released by the metal is -312.48 J
For part B :
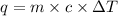
q = heat released by the metal = -312.48 J
m = mass of metal = 43.1 g
c = specific heat of metal = ?
 = change in temperature =
= change in temperature =
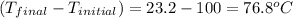
Now put all the given values in the above formula, we get:
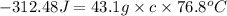
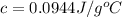
Thus, the specific heat of the metal is