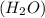Answer : The correct option is, (D) 1 mole of water
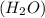
Explanation :
As we know that, 1 mole of substance contains
 number of atoms.
number of atoms.
(A) 1 mole of methane
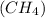
As, 1 mole of methane contains 3 moles of hydrogen atoms.
So, 1 mole of methane contains
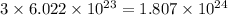 of hydrogen atoms.
of hydrogen atoms.
The number of hydrogen atoms present in 1 mole of methane are
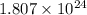
(B) 2 moles of ammonia
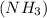
As, 1 mole of ammonia contains 3 moles of hydrogen atoms.
So, 2 mole of ammonia contains
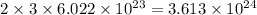 of hydrogen atoms.
of hydrogen atoms.
The number of hydrogen atoms present in 1 mole of ammonia are
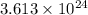
(C) 2 moles of hydrogen gas
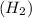
As, 1 mole of hydrogen gas contains 2 moles of hydrogen atoms.
So, 2 mole of hydrogen gas contains
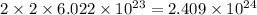 of hydrogen atoms.
of hydrogen atoms.
The number of hydrogen atoms present in 1 mole of hydrogen gas are
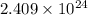
(D) 1 mole of water
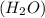
As, 1 mole of water contains 2 moles of hydrogen atoms.
So, 1 mole of water contains
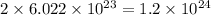 of hydrogen atoms.
of hydrogen atoms.
The number of hydrogen atoms present in 1 mole of water are
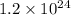
Hence, the correct option is, (D) 1 mole of water